Average Cost To Build An Addition. The average cost to build a home addition or add a room is $48,000, with most homeowners spending between $22,500 and $74,000.A room addition costs from $86 to $208 per square foot depending on the room size, materials, labor, location, and if your building-up or out. When you have just about any questions with regards to exactly where in addition to how you can make use of metal projects ideas, you'll be able to e mail us in the web page. Below are 22 best pictures collection of room addition floor plans photo in high resolution. We have addition exercises for all grade levels, from basic equations using 1-20 to missing factors and word problems. We also have formats to appeal to all types of learners: Students needing help with addition can count along a number line, run though addition facts, use visual aids for assistance, or just do some simple equations to make.
Summary: The cost of a home addition compares to the cost of building a small house. The cost of a room addition can be less if you remodel an existing area such as a basement, garage, porch, or attic.
Carl, we are thinking of adding a home addition to our present house. Where do we start? Should we call a remodeling contractor to get ideas and plans? We need more living space! Help! Sue
Hi Sue,
You need to have a good idea how much it will cost to build an addition before you get too far along in the process.
The cost per square foot to build such a relatively small living space is higher than most people expect it to be. I explain why below, but first let’s see just how much higher we are talking about.
Here’s a nice home addition plan from CADSmith Studio
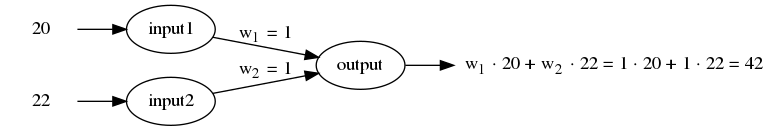
This 22’ x 22’ addition is suitable for a traditional 2 story or cape style home.
1st floor living space: 484 sq. ft. (22 x 22)
2nd floor living space: 242 sq. ft. (22 x 11)
Total = 726 sq. ft.
Addition House Plan courtesy of CADSmith Studio
I ran a cost analysis using the cost to build software that is on Getting Started to determine the cost of this home addition plan.
Using Grand Rapids, MI as a random city, I came up with a rough estimated cost to build the addition of $72,214, or $100 per square foot.
This approximate cost to build is current for 2019 and includes a General Contractor’s markup of $8,301. By using that same cost estimating software, you can run your own approximate cost estimate for your house addition using your city and state and your own construction parameters. It’s easy to use.
Always keep in mind that the cost to build house additions or a new home can vary considerably depending on design, quality of materials selected, and on actual bids for labor and materials. Geographical location affects cost too.
NOTE: Many locales have added impact fees on top of construction permits. The costs of these fees can be expensive. Investigate them early on in your home building planning. They are NOT included in these cost estimates.
As you can see, building a house addition is like building a small house, and just as in building a new home, you can act as your own General Contractor and estimate the costs based on you hiring your own building contractors or subcontractors (tradespeople) and buying the materials needed.
The basic difference in estimating a home addition vs. a new home will be the increase of some square footage building costs.
This is due to the smaller size of the job. Subcontractors may want a few more dollars for their labor because they could be earning more on a larger job for almost the same amount of labor time.
On the other hand, the general contractor (that you won’t be hiring) would probably have charged a higher markup for the same reason, so you will save there too.
As I mention in my book, the National Association of Home Builder recommends that professional builders aim for a 50 percent gross profit margin on the cost of home additions and remodeling! The savings you realize by being your own general contractor will more than offset the increase in labor costs from subs.
As for calling a remodeling contractor for plans and/or ideas, you certainly should. Get bids from at least three remodeling contractors. Be sure to clearly state your budget to them. Be sure to get at least three references and CHECK THOSE REFERENCES!
I would also consult with a local design firm. You can find them with a search for “(Your City) Home Designing and Planning.” Explain your budget to the home designer. As professional designers they should know how to design a home addition plan to fit your budget. Again, get at least three references and CHECK THEM!
You should make a list of all the materials the project will need and use a cost estimate spreadsheet as a starting point. Obtain competitive bids as though you were building a house and proceed from there.
Don’t add too much value to your home because all houses need to be sold sooner or later, and you don’t want to have the most expensive house in the neighborhood when you put it on the market. It’s a real estate fact of life: The most expensive house in the neighborhood is very difficult to sell.
Before even deciding to go ahead with the addition, get an appraisal or market analysis from a Realtor as to the completed value, or the value of the addition added. If you are getting a loan, your mortgage lender will have an appraisal done before deciding to lend you money.
Keep in mind that making existing nonliving spaces habitable may be the least expensive way to add more living space.
After all, these areas may already have a roof, foundation, floors, and some or all of the exterior walls.
Since you are considering building an addition, check to see that you have room on your lot to do so. Chapter 8ms. mas website.
All local zoning laws and most property deed restrictions have minimum foundation setback requirements that cannot be violated.
These setback requirements usually require a minimum distance that the foundation (or roof overhang) can be from side property lines, front and rear property lines, easements, streets, and neighboring houses.
Check with your local building inspection department and, if you have one, your homeowners association.
Also check your deed restrictions. These deed restrictions are available as public records at your county register of deeds office.
A mortgage report survey shows exactly where the foundation footprint of your house is in relation to the required setbacks.
If you don’t have an existing mortgage report survey, it is well worth the money to get one. Call a few local land surveyors to determine the current cost of this type of land survey.
Once you get plans for your home addition, it should be first drawn onto the survey to be sure it fits. If it fits on paper, it will fit in reality. If it violates setbacks, adjust your home addition plan accordingly.
Typical Mortgage Report Survey
As for “where do you start”, you always start with a budget.
Budget = cash + borrowing power.
For borrowing power, talk to a mortgage lender of your choice.
Good luck, Carl Heldmann
Related Pages
Contributors
Prof. Steven Farmer (Sonoma State University)
- Gamini Gunawardena from the OChemPal site (Utah Valley University)
General reaction

Example
Mechanism
1) Nucleophilic attack by hydroxide
2) Leaving group removal
3) Deprotonation
Contributors
Prof. Steven Farmer (Sonoma State University)
Alkaline hydrolysis of amides
In alkaline hydrolysis the amide is heated with boiling aqueous sodium or potassium hydroxide. The nucleophilic hydroxide ion adds to the carbonyl carbon to form a tetrahedral intermediate, which, with the help of the aqueous solvent, expels the nitrogen as the free amine:
Reduction of carboxylic acids and their derivatives
Reduction of acid chlorides and esters
Acid (acyl) chlorides can be converted to aldehydes using lithium tri-tert-butoxyaluminium hydride (LiAlH(Ot-Bu)3). The hydride source (LiAlH(Ot-Bu)3) is a weaker reducing agent than lithium aluminum hydride. Because acid chlorides are highly activated they still react with the hydride source; however, the formed aldehyde will react slowly, which allows for its isolation.
General reaction:
Example
Acid chlorides can be converted to aldehydes using lithium tri-tert-butoxyaluminium hydride (LiAlH(Ot-Bu)3). The hydride source (LiAlH(Ot-Bu)3) is a weaker reducing agent than lithium aluminum hydride. Because acid chlorides are highly activated they still react with the hydride source; however, the formed aldehyde will react slowly, which allows for its isolation.
General Reaction:
Example
Esters can be converted to aldehydes using diisobutylaluminium hydride (DIBAH). The reaction is usually carried out at -78 oC to prevent reaction with the aldehyde product.
Example
Esters can be converted to 1o alcohols using LiAlH4, while sodium borohydride ($$NaBH_4$$) is not a strong enough reducing agent to perform this reaction.
Example
Mechanism
1) Nucleophilic attack by the hydride
22: A Simple Addition Anchor Chart
2) Leaving group removal
3) Nucleophilic attack by the hydride anion
4) The alkoxide is protonated
Going from reactant to products simplified
Example
Reduction of amides using LiAlH4.
General Reaction
Example: Amide Reduction
Alkyl groups attached to the nitrogen do not affect the reaction.
Mechanism
1) Nucleophilic attack by the hydride
2) Leaving group removal
3) Nucleophilic attack by the hydride
Contributors
- Prof. Steven Farmer (Sonoma State University)
Reduction of acid chlorides and esters using LiAlH4
The mechanism of action of hydride reductions on acid chlorides and esters (carboxyl groups) is similar to that taking place with carbonyl compounds, except that acid chlorides and esters have a leaving group (–Cl and –OR). So the reaction does not stop at formation of the alkoxide ion as a tetrahedral intermediate, but keeps going with an internal nucleophilic displacement of the leaving group. The direct outcome of this process is formation of the corresponding carbonyl compound (aldehyde or ketone), which may or may not undergo further reduction to alcohol, depending on the nature of the reagents used and reaction conditions. The following mechanism illustrates this concept.
For simplicity, only the hydride ion is shown. If a full reactivity reducing agent such as $$LiAlH_4$$ is used, the reaction does not stop at the aldehyde stage, since the carbonyl carbon of the aldehyde can be attacked by another hydride equivalent. This results in formation of the primary alcohol (after hydrolysis of the alkoxide ion) as the final product.
The net reaction then is:
22: A Simple Addition Worksheets
The reaction with an ester is similar, but the leaving group is different (R’O–). Can you draw the mechanism that leads to formation of the products shown?
Notice that with both (and all) carboxyl groups, hydride reductions lead to formation of primary alcohols only. There is no possibility of forming secondary alcohols by this method because the carboxyl group is at the end of the carbon chain, or else the chain gets broken so that the carboxyl carbon ends up at the end of a chain in the final product.
Nucleophilic acyl substitution reactions of carboxylic acids
Acid anhydride formation
An acid anhydride (or just anhydride) is the product of formal condensation of two oxoacid molecules with the release of a water molecule. The most common anhydrides in organic chemistry are those derived from carboxylic acids at high temperatures to remove water.
Carboxylic acids react with thionyl Chloride ($$SOCl_2$$) to form acid chlorides
During the reaction the hydroxyl group of the carboxylic acid is converted to a chlorosulfite intermediate making it a better leaving group. The chloride anion produced during the reaction acts a nucleophile.
Example
Mechanism
1) Nucleophilic attack on Thionyl Chloride
2) Removal of Cl leaving group
3) Nucleophilic attack on the carbonyl
4) Leaving group removal
5) Deprotonation
Conversion of carboxylic acids to amides
The direct reaction of a carboxylic acid with an amine would be expected to be difficult because the basic amine would deprotonate the carboxylic acid to form a highly unreactive carboxylate. However when the ammonium carboxylate salt is heated to a temperature above 100 oC water is driven off and an amide is formed.
General Reaction
Going from reactants to products simply
Conversion of carboxylic acids to amide using DCC as an activating agent
The direct conversion of a carboxylic acid to an amide is difficult because amines are basic and tend to convert carboxylic acids to their highly unreactive carboxylates. In this reaction the carboxylic acid adds to the DCC molecule to form a good leaving group which can then be displaced by an amine during nucleophilic substitution. DCC induced coupling to form an amide linkage is an important reaction in the synthesis of peptides.
Basic reaction
Going from reactants to products simplified
Mechanism
1) Deprotonation
2) Nucleophilic attack by the carboxylate
3) Nucleophilic attack by the amine
4) Proton transfer
5) Leaving group removal
Chemistry of Amides
Nitriles can be converted to amides. This reaction can be acid or base catalyzed
Carboxylic acid can be converted to amides by using DCC as an activating agent
Direct conversion of a carboxylic acid to an amide by reaction with an amine.
Acid chlorides react with ammonia, 1o amines and 2o amines to form amides
Acid anhydrides react with ammonia, 1o amines and 2o amines to form amides
Conversion of amides into carboxylic acids: Hydrolysis
This page describes the hydrolysis of amides under both acidic and alkaline conditions. It also describes the use of alkaline hydrolysis in testing for amides.
What is hydrolysis?
Technically, hydrolysis is a reaction with water. That is exactly what happens when amides are hydrolyzed in the presence of dilute acids such as dilute hydrochloric acid. The acid acts as a catalyst for the reaction between the amide and water. The alkaline hydrolysis of amides actually involves reaction with hydroxide ions, but the result is similar enough that it is still classed as hydrolysis.
Hydrolysis under acidic conditions
Taking ethanamide as a typical amide. If ethanamide is heated with a dilute acid (such as dilute hydrochloric acid), ethanoic acid is formed together with ammonium ions. So, if you were using hydrochloric acid, the final solution would contain ammonium chloride and ethanoic acid.
[ CH_3CONH_2 + H_2O + HCl rightarrow CH_3COOH + NH_4^+Cl^-]
Hydrolysis under alkaline conditions
Also, if ethanamide is heated with sodium hydroxide solution, ammonia gas is given off and you are left with a solution containing sodium ethanoate.
[ CH_3CONH_2 + NaOH rightarrow CH_3COONa + NH_3]
Conversion of amides into amines: Reduction
Amides can be converted to 1°, 2° or 3° amines using LiAlH4.
Examples
Amide Reductions
Alkyl groups attached to the nitrogen do not affect the reaction.
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
- Jim Clark (Chemguide.co.uk)
Grignard reagents convert esters into tertiary alcohols
As we saw in section 20.3, the addition of Grignard reagents converts esters to 3o alcohols. In effect the Grignard reagent adds twice. The initial steps of the mechanism involve a nucleophilic acyl substitution to form a ketone, which then reacts with the second mole of the Grignard reagent.General Reaction
Examples
Contributors
Prof. Steven Farmer (Sonoma State University)
Bohemian Violin Expansion 2 by Virharmonic is a Virtual Instrument Audio Plugin and Soundware (e.g. Samples or presets that load into other products) for macOS and Windows. It includes, and is therefore 'powered by', UVI Workstation, which functions as a VST Plugin, an Audio Units Plugin and an AAX Plugin. Vst violin plugin. Virtual Violin, VST, AU, AAX, Solo Violin - Virharmonic. The Bohemian Violin was created to free you to perform real Violin Performances fast, without the need to be a violinist yourself. Internet Connection (for download and activation) Hard Drive (HDD) 7200 rpm. BOHEMIAN is a diverse collection of 800+ unique traditional, and morphed ethnic instruments & multis. This groundbreaking library is derived entirely from recordings of over a dozen unique bohemian street instruments ranging from the Hang Drum, to a Harmonic Tube, to a Didgeridoo, and beyond.
Earlier, we examined the aldol reactions as a nucleophilic carbonyl addition in section 20.6, in which the electrophile is the carbonyl carbon of an aldehyde or ketone. A nucleophilic enolate can also attack the carbonyl carbon of a carboxylic acid derivative in a nucleophilic acyl substitution reaction.This is referred to as a Claisen condensation, after the German chemist Ludwig Claisen (1851-1930).Basic reaction
Going from reactants to products simply
Example: Claisen Condensation
1) Enolate formation
2) Nucleophilic attack
3) Removal of leaving group

Dieckmann Condensation
A diester can undergo an intramolecular reaction called a Dieckmann condensation.
Example: Dieckman Condensation
Crossed Claisen condensation
Claisen condensations between different ester reactants are called CrossedClaisen reactions. Crossed Claisen reactions in which both reactants can serve as donors and acceptors generally give complex mixtures. Because of this most Crossed Claisen reactions are usually not performed unless one reactant has no alpha hydrogens.
Example : Crossed Claisen Condensation
Contributors
- Prof. Steven Farmer (Sonoma State University)
